Overview
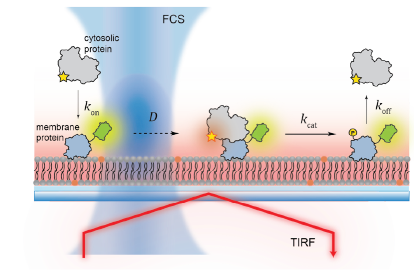
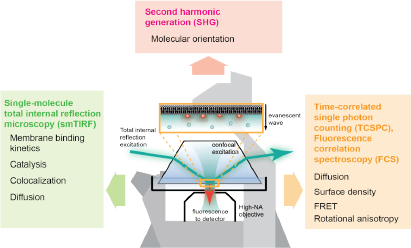
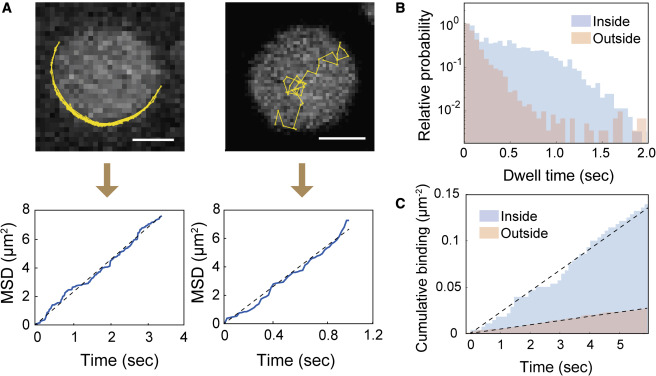
For survival, cells rely on complex networks of biochemical reactions. Even though the spatiotemporal control of biochemical reactions on lipid membranes is an important aspect of cellular processes, much of it has been inaccessible by conventional solution-based biochemical methods. In order to answer questions about lipid membranes, we use reconstituted membrane systems, such as supported lipid bilayers (SLBs), giant unilamellar vesicles (GUVs), and small unilamellar vesicles (SUVs), and probe the membranes with fluorescence microscopy techniques, such as total internal reflection fluorescence (TIRF) microscopy, confocal fluorescence microscopy, fluorescence correlation spectroscopy (FCS), time-correlated single-photon counting (TCSPC). These techniques allow us to make quantitative measurements about membrane processes, such as ligand binding and dimerization, diffusion, and lipid oxidation.
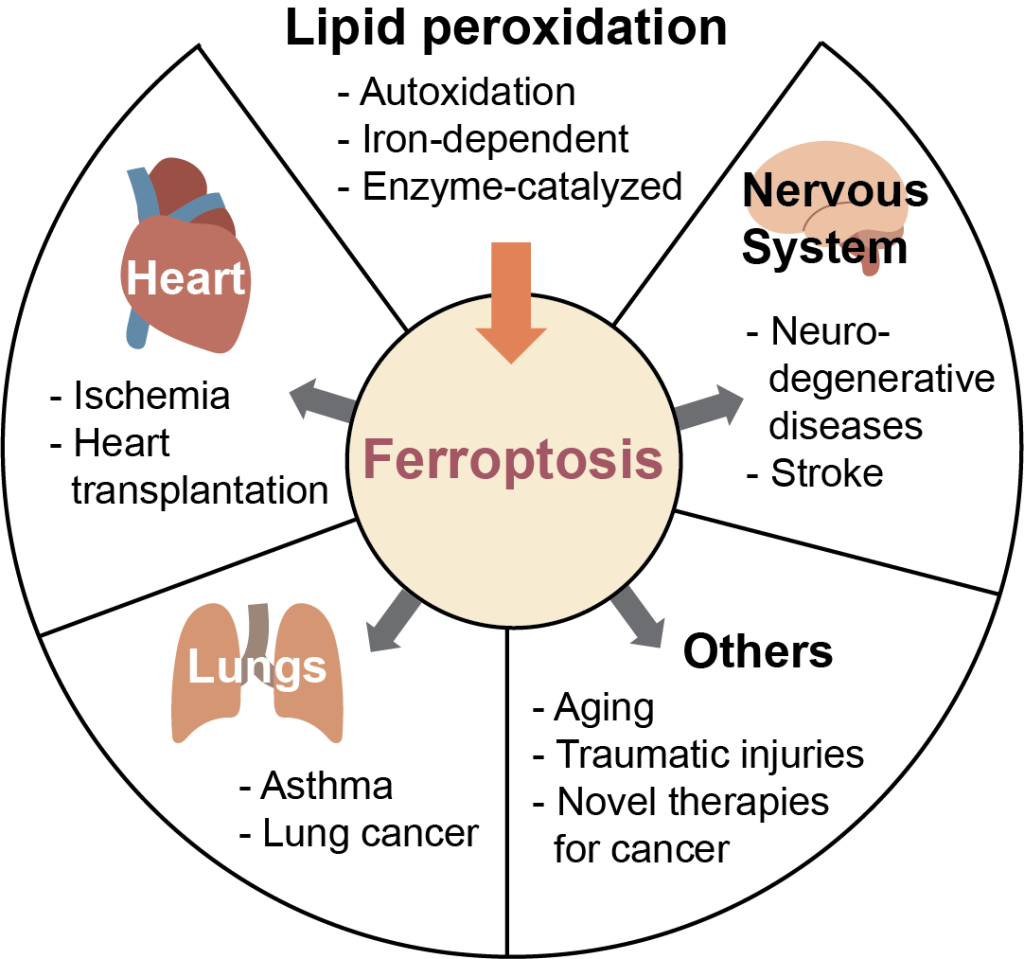
Mechanisms and kinetics of membrane lipid peroxidation
Lipid peroxidation is associated with a legion of health concerns such as Alzheimer’s and Parkinson’s diseases, cancer, cardiovascular diseases, and numerous others. However, the link between these pathologies and lipid peroxidation is currently unclear. A newly discovered type of regulated cell death, ferroptosis, occurs by the accumulation of lipid peroxides in cells, and may be the link between lipid peroxidation and disease. Fluorescent probes measure this process and are used to investigate lipid peroxidation kinetics in membranes.
Molecular assembly of multidomain proteins on membranes
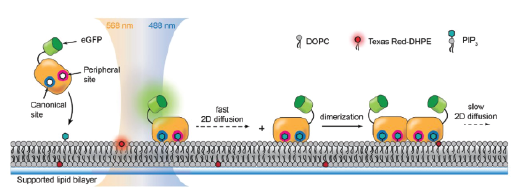
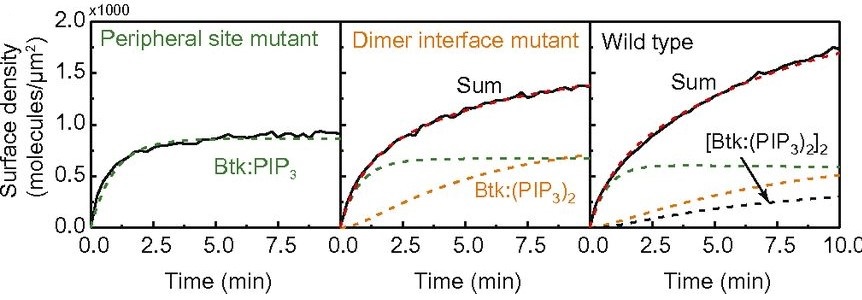
Bruton’s tyrosine kinase (Btk) is a cytoplasmic tyrosine kinase that plays a central role in B cell signal activation and signal transduction. However, the active form of Btk requires localization to the plasma membrane – a process mediated by a lipid second messenger phosphatidylinositol (3-5)-triphosphate (PIP3). Upon binding PIP3 via the pleckstrin homology (PH) domain of the protein, Btk undergoes conformational changes, dimerization, and activation. Interestingly, while the PH domain facilitates dimerization and activation of the protein, it may also facilitate the formation of 2-dimensional ordered structures much larger than protein dimers on the membrane, mediating a process known as biological phase separation. We hope to more fully understand the structures, kinetics, and stoichiometry of this process through the use of our reconstituted membrane systems and microscopy techniques.
Membrane active peptides
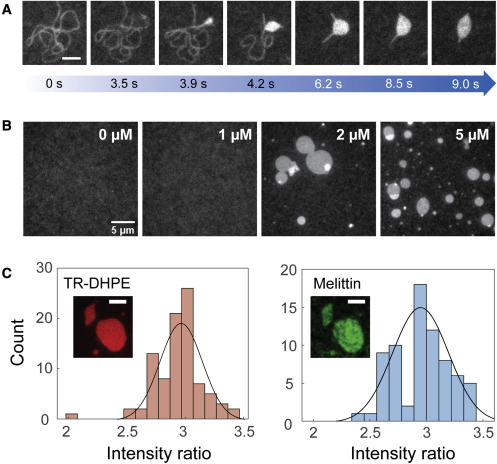
Melittin and phospholipase A2 (PLA2) , the principal components of bee venom, take advantage of altered membrane structures for their function—the disruption of the cellular membranes—which is responsible for the pain and inflammation around a bee sting. A synergistic effect in the cell lysis rates between melittin and PLA2 has been previously observed. Using the SLB system and TIRF microscopy, the synergistic mechanism of melittin and PLA2 was investigated. PLA2 was shown to preferentially interact with melittin-induced deformations on the membrane. Our experimental systems will be employed to further investigate this system.
